________________________________
Light emitted as ______________
(___________ ___________)
II. Explaining photoelectric effect using quantum theory
A. ______________ Theory
1) atoms can only _____________ or
________ ______________ in ___________
amounts called __________ (___________________)
2) Energy of each quanta is proportional to frequency of
radiation
|
|
h = plancks constant = _____________________
E
= ________
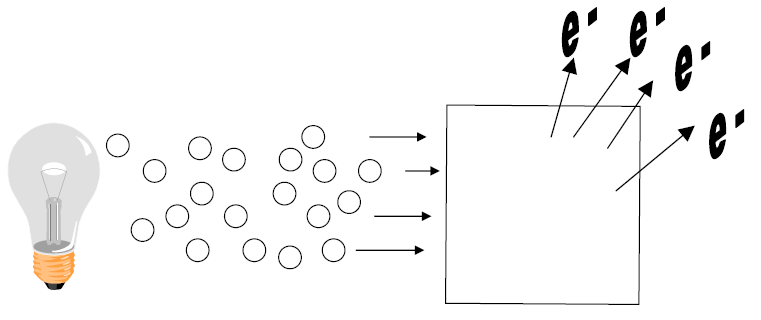
B) Einstein explains photoelectric effect
1) Relationship between _____________________ and ________________ explained.
| ________________________________ |
________________________________ |
|
|
|
|
|
|
|
Bright Light
|
Dim Light |
|
|
|
|
(_______________________) |
(_______________________) |
|
______ __________________ Light |
______ __________________ Light |
|
|
|
|
(_______________________) |
(_______________________) |
Brightness - measure of __________________________________
Frequency - measure of
__________________________________
|
|
|
| Photon Energy = 5 eV |
KE = 2 eV |
** eV - _______________ - unit of _______________
How much work did this photon have to do to remove the electron?
Answer - _________