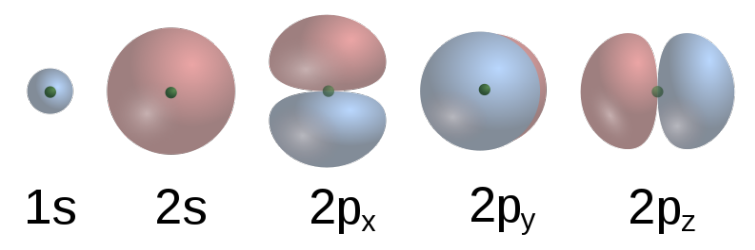|
Bohr Limitations
Do you remember what causes
The Aurora from TSO Photography on Vimeo. The Aurora from Terje Sorgjerd on Vimeo.
C) Limitations of Bohr Model
1) electron couldn’t circle around nucleus like a planet! …
Because they would lose energy (by emitting electromagnetic radiation) & spiral into nucleus.
2) Bohr was not able to explain electron orbits of large atom w/ many electrons
Uncertainty Principle Wave Function And Wave-Particle Duality
Deutsches Bundesarchiv (German Federal Archive), Bild183-R57262 Werner Heisenberg
When you are studying sub-atomic particles, you change them.
(A particles position and momentum are affected by your measuring devices.)
School blocks YouTube? Click below.
The more you know
Present Theory of the Atom
Electrons do not have precisely described positions and momenta
The 'probable' position and momenta are described mathematically
Electrons occupy regions in space called 'clouds'. Sven Benutzer:Benji.
Spectral Lines - Electron Cloud Model
Enrichment Quantum Mechanics: The Uncertainty Principle
Spectroscope
Science of the
©Tony Mangiacapre., - All Rights Reserved [Home]
|
|||||