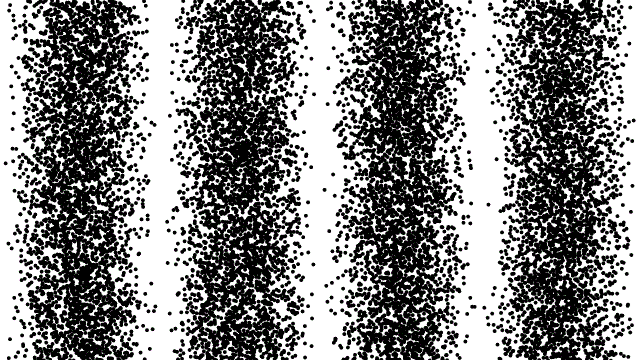|
Matter Waves
Show Me The Physics
D) Matter Waves - ALL moving particles have wave properties.
Louis de Broglie
p = momentum of the particle λ - called DeBroglie Wavelength h - Planck's constant
The wavelength of ordinary objects are insignificant because
Electrons are small enough to have a significant
1927- Diffraction and interference patterns were observed for electrons.
A scientist passed electrons through tiny double slits and observed interference patterns
Where did they get slits small enough 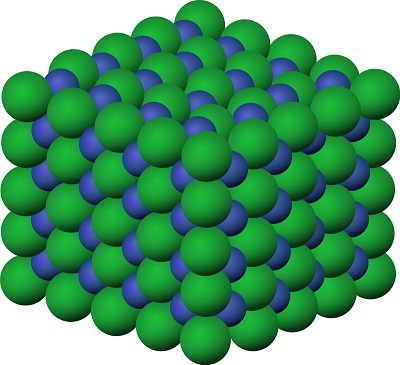
Pattern produced when a beam of electrons pass through a double slit
When you pass a beam of electrons through the slits of a crystal you get an interference pattern
Final Summary
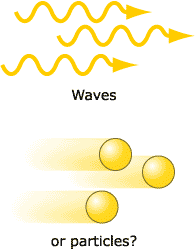
IS LIGHT A WAVE OR A PARTICLE?
- Douglas Hofstadter
©Tony Mangiacapre., - All Rights Reserved
[Home] |
||||||