|
Black Box
showmethephysics.com
Background Electrons give off light when they return to the ground state after being excited by an energy source
Bohr studies the light given
Neils Bohr, right
'Black Body' = heated body that gives off only the light from its internal glow - no reflected light
Material was heated and the light
Spectroscopy
School blocks YouTube? Click below St. Mary's U. Astronomy and Physics Dept.
Spectral lines
Emission Spectra
Whatever colors an element gives off, it also .....
... absorbs
Atomic Spectra
Atomic Spectra for Iron
Full Spectrum
Hydrogen Emission
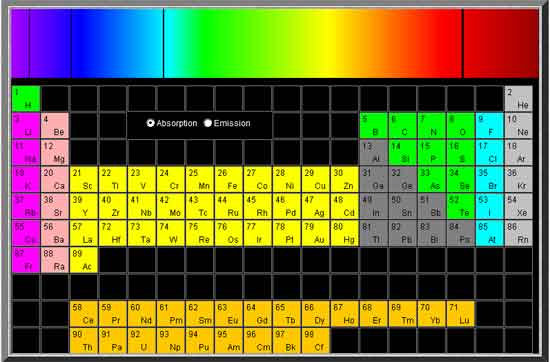
Place your mouse over the image to see the absorption spectra
Full Spectrum
Helium Emission/Absorption
Place your mouse over the image
Every Energy level transition creates a single spectral line
| Onsite Version |
"Science of the Silicon Solar Cell" Don Ion
How many different spectral
lines can be produced
Answer
10 Spectral Lines
10 colors
10 frequencies 10 photons
Black Body Radiation Results:
1. Only the high energy objects gave off colors on the violet side of the spectrum
2. Electrons in atoms are not capable of giving off all colors (energies)
3. Each element gives off a unique pattern of colors called a 'bright line spectrum'.
Answer 1. Spectral lines used to:
To Identify an Unknown Element
- Electrify, Heat it up and analyze its colors.
- Compare emitted colors to a database of elemental spectral lines
The diagram below represents the bright-line spectra of four elements, A, B, C, and D, and the spectrum of an unknown gaseous sample.
Based on comparisons of these spectra,
B and C
What are stars made of ?
ŠTony Mangiacapre., - All Rights Reserved [Home] Established 1995 Use any material on this site (w/ attribution) |
||||||||||||||||||||||||||||