![]()
![]()

Bohr Examples
|
What parts of the atom move? NOVA scienceNOW | Amazing Atoms | PBS"Here is the latest, atomically correct, version of our old friend, the atom." School blocks YouTube? Use file below. |
showmethephysics.com
Ex) How much energy is absorbed when an electron in hydrogen moves from the energy levels n = 2 to n = 5?
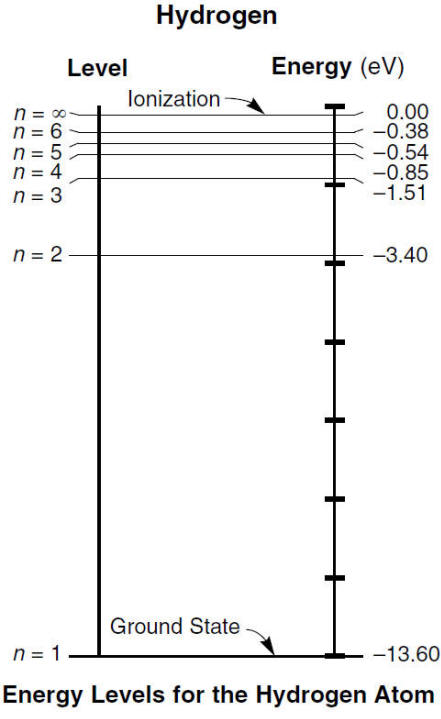
Subtract the absolute values to find the energy needed to be absorbed
Answer
3.4 eV - .54 eV = 2.86 eV
Ex) How much energy is needed for an electron in the n = 1 energy level to move to the n = 2 energy level?
n = 1 -13.6 eV
n = 2 -3.40 eV
Throw out the
negative signs and subtract
= 13.6 eV - 3.4 eV
= 10.2 eV Absorbed
Frequency of photon absorbed
E = hf
h = 6.63 x 10-34 Js
E = 10.2 eV
Convert eV to Joules
page 1 Reference
1 eV = 1.6 x 10-19 J
E = 10.2 eV x 1.6 x 10-19 J
= 1.6 x 10-18 Joules
E = hf
1.6 x 10-18 Joules =
(6.63 x 10-34 Js) f
f = 2.4 x 1015 Hz
Ex) Which energy level transition in MERCURY will produce a photon emission of .22 ev?
c to b transition
(Difference in energy
between b and c is .22 eV)
5) Ionization Energy - minimum energy needed to remove an electron from an atom.
Ionization Energy - greater than or equal to energy at that level.

Ionization Energy of Hydrogen
n = 1
13.6 eV or greater
n = 2
3.4 eV or greater
n = 3
1.51 eV or greater

Ex) A photon having an energy of 15.5 electron volts is incident upon a hydrogen atom in the ground state.
The photon may be absorbed by the atom and
a) ionize the atom
b) excite the atom to n = 3
c) excite the atom to n = 2
d) excite the atom to n = 4

a) ionize the atom
Bohr Energy Transitions / Ionization
![]()
 |
Electrons
Energies? |
 |
![]()
ŠTony Mangiacapre., - All Rights Reserved
[Home]
Established 1995
Use any
material on this site (w/ attribution)