![]()
![]()

Explaining the
Photoelectric Effect
|
What is the relationship between E and f?
|
II. Explaining photoelectric effect using quantum theory

A. Quantum Theory
1) electrons can only absorb or emit energy in discrete amounts called quanta (packets)
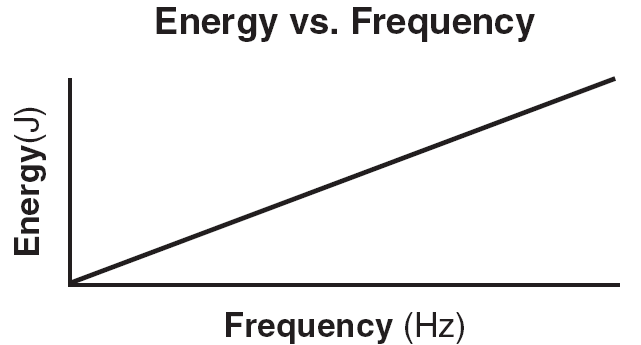
2) Energy of each quanta (photon) is proportional to frequency of radiation
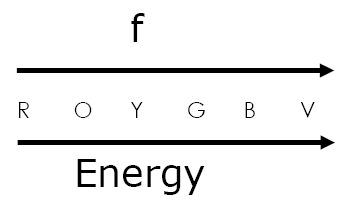
Energy of a Quanta
|
E = hf |
June 2004 #40
 Max Planck |
h = Planck's constant =6.63 X 10-34 Js |
Since: c = fλ
c = 3 x 108 m/s
And f = c/λ
|
|
 |
Einstein, Photoelectric Effect |
 |
ŠTony Mangiacapre.,- All Rights Reserved
[Home]
Established 1995
Use any
material on this site (w/ attribution)